Abstract
Background: Ruxolitinib (rux) is a JAK1/2 inhibitor approved for the treatment of intermediate and high risk myelofibrosis (MF) patients with constitutional symptoms and/or symptomatic splenomegaly. Unfortunately, many patients discontinue rux due to lack or loss of initial response, or treatment related cytopenias. Optimal management of MF after rux discontinuation is not clear. In this study, we aimed to further investigate rux discontinuation; the causes that lead to it, treatment approaches after, and ultimate clinical outcomes.
Methods: We retrospectively reviewed MF patients who presented to our institution between 8/2008 and 3/2016. We included rux-treated patients who discontinued treatment after ≥ 4 consecutive months (mo) of treatment and who had ≥ 5 mo of follow-up after discontinuation. Overall survival (OS) was defined from time of rux discontinuation. Patients who underwent allogeneic transplant (allo-HCT) were censored at time of transplant. Response to rux was defined by IWG criteria.
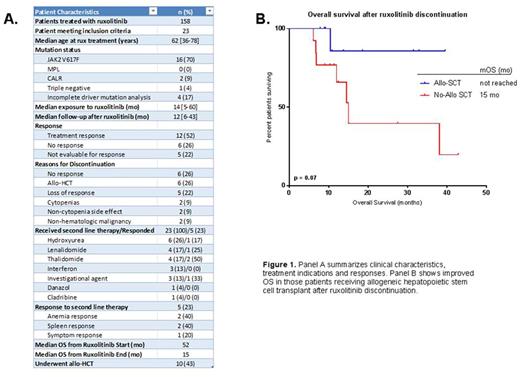
Results: Out of 158 MF patients treated with rux, 23 (14.6%) met inclusion criteria. The median age at rux initiation was 62 years. Sixteen (70%) patients had a JAK2 V617F mutation, 2 (9%) had a CALR mutation, 1 (4%) was triple-negative (negative for JAK2 V617F, MPL and CALR mutations) and 4 (17%) had incomplete analysis to determine driver mutation status. The median exposure to rux prior to discontinuation was 14 mo [5-60 mo]. Median time of follow-up after rux discontinuation was 12 mo [6-43 mo]. Among the 23 patients, 12 responded to rux, 6 had no response and 5 were not evaluable for response. Among the 12 responders, mean duration of rux treatment prior to discontinuation was 29.4 mo compared to 10.4 mo in the 6 non-responders (p = 0.03). The most common reasons for discontinuation were lack of efficacy (26%), decision to undergo allo-HCT (26%), or progression of symptoms after initial response (22%). Less common reasons for discontinuation included development of cytopenias (9%), side effects unrelated to cytopenias (9%), and diagnosis of a non-hematologic malignancy requiring treatment (9%).
Of 23 evaluable patients, 100% received a second line of therapy after rux discontinuation. Five patients (28%) received >1 salvage therapy. The most common treatment after rux discontinuation was allo-HCT (n = 10, 43%). Apart from transplant, treatment choices after rux discontinuation included hydroxyurea (26%), lenalidomide (17%), thalidomide (17%), interferon (13%), investigational agents (13%), danazol (4%) and cladribine (4%). Rux was re-initiated in one patient who did not respond. The response rate to subsequent lines of therapy was 23% (n=5). Of five documented responses, two were anemia responses after treatment with thalidomide, one was a spleen response with lenalidomide, one a symptom response with hydroxyurea, and one a spleen response after treatment with an investigational JAK2/FLT3 inhibitor (SB1518).
Median OS from rux initiation was 52 mo, whereas median OS after rux discontinuation was 15 mo. Median OS was 52 mo vs. 29 mo in patients who responded to rux compared to non-responders, respectively (p = 0.27). After rux discontinuation, those who underwent allo-HCT had longer OS compared to those who did not (median OS not reached vs 15 mo, OR 0.18 [0.04-0.73]; p = 0.07). Response to subsequent lines of therapy was not associated with improved OS (p = 0.36).
Molecular profiling was available for 14/23 patients. In 13 cases, next generation sequencing (NGS) was performed prior to initiation of rux. Thirteen (93%) patients were found to have at least one somatic mutation, while 3 patients had ≥ 3 mutations. In patients with 0-2 somatic mutations, there was an 88% response rate to rux compared with a 67% response rate in patients with ≥ 3 mutations (p = 0.13). ASXL1 mutation was found in 8 (62%) patients. All other mutations occurred with a frequency ≤ 15%. Of the two patients with NGS data who did not respond to rux, both had ASXL1 mutations. ASXL1 mutation status did not correlate with survival; however 5 (63%) patients with ASXL1 mutations underwent allo-HCT.
Conclusion: A significant percentage of rux-treated patients discontinue treatment due to lack or loss of response. After rux discontinuation, anemia, spleen and symptom responses are infrequent. Allo-HCT offers the best chance for long-term survival. Outcome after rux failure is poor and represents an unmet need in the treatment of MF.
Padron: Incyte: Honoraria, Research Funding. Sallman: Celgene: Research Funding. Lancet: Erytech: Consultancy; Celgene: Consultancy; Pfizer: Other: Institutional research funding, Research Funding; Jazz Pharmaceuticals: Consultancy; Boehringer Ingelheim: Consultancy; BioSight: Consultancy; Novartis: Consultancy; Bio-Path Holdings: Consultancy; Janssen: Consultancy. Komrokji: Celgene: Honoraria; Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal